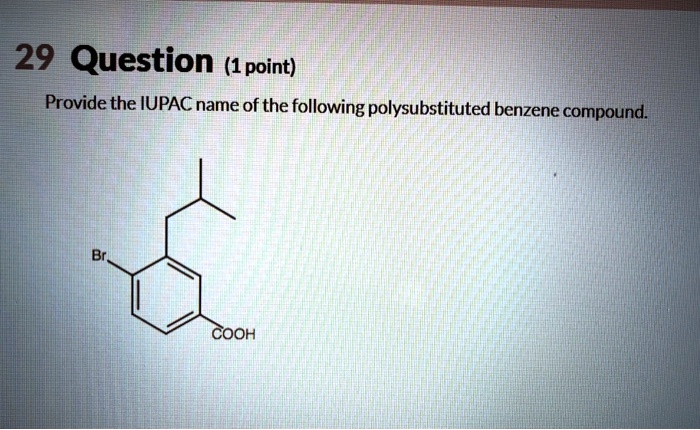
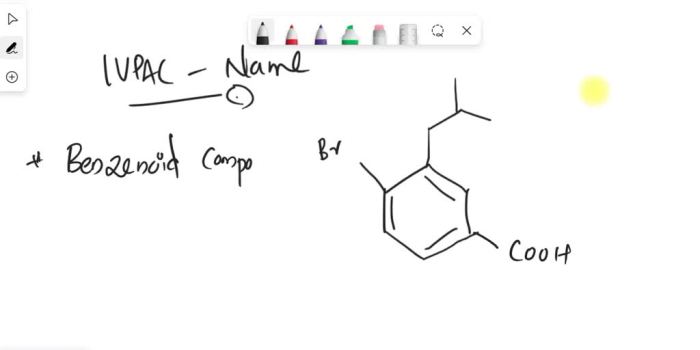
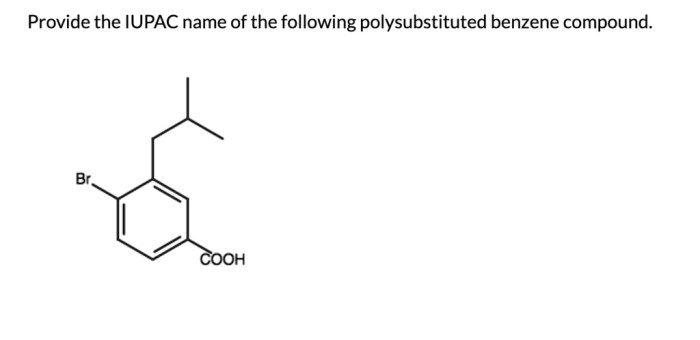
Provide an acceptable IUPAC name for the following polysubstituted benzene. The International Union of Pure and Applied Chemistry (IUPAC) has established guidelines for naming organic compounds, including polysubstituted benzenes. These guidelines ensure consistency and clarity in chemical nomenclature. Understanding the IUPAC naming conventions is essential for effective communication and accurate identification of these compounds.
This guide will delve into the IUPAC naming basics, including rules for identifying substituents, numbering the benzene ring, and constructing the IUPAC name. It will also address exceptions and special cases to the IUPAC naming rules. By following these guidelines, chemists can confidently assign IUPAC names to polysubstituted benzenes.
IUPAC Naming of Polysubstituted Benzenes: Provide An Acceptable Iupac Name For The Following Polysubstituted Benzene
In organic chemistry, the International Union of Pure and Applied Chemistry (IUPAC) provides guidelines for naming organic compounds in a systematic and consistent manner. For polysubstituted benzenes, which are benzene rings with multiple substituents, specific rules apply to ensure clear and unambiguous naming.
1. IUPAC Naming Basics, Provide an acceptable iupac name for the following polysubstituted benzene
The IUPAC naming rules for polysubstituted benzenes follow a specific set of conventions:
- The base name of the compound is benzene.
- Substituents are named as prefixes to the benzene name.
- Substituents are listed in alphabetical order.
- Numbers are used to indicate the positions of substituents on the benzene ring.
2. Identifying Substituents
Substituents on a benzene ring can be classified into different types:
- Alkyl groups(e.g., methyl, ethyl)
- Haloalkyl groups(e.g., chloromethyl, bromopropyl)
- Aryl groups(e.g., phenyl, naphthyl)
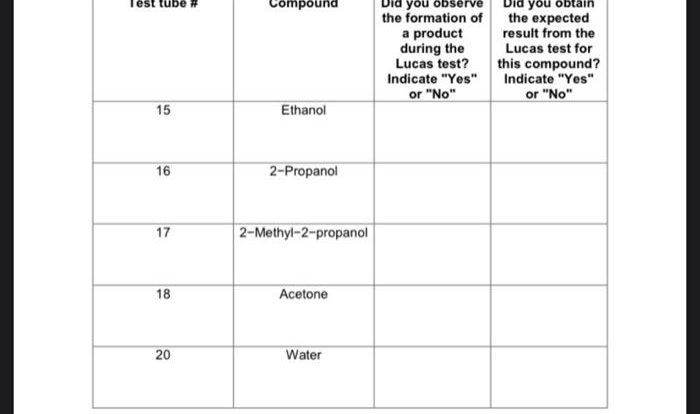
- Hydroxy groups(OH)
- Amino groups(NH2)
- Carboxylic acid groups(COOH)
Priority rules are used to assign prefixes to substituents. The priority order is based on the following factors:
- Atomic number: Substituents with higher atomic numbers have higher priority.
- Degree of unsaturation: Substituents with more double or triple bonds have higher priority.
- Alphabetical order: If all other factors are equal, substituents are listed in alphabetical order.
3. Numbering the Benzene Ring
The benzene ring is numbered to indicate the positions of substituents. The preferred starting point for numbering is the carbon atom attached to the highest priority substituent.
If there are multiple substituents with equal priority, the ring is numbered to give the lowest possible numbers to the other substituents.
4. Constructing the IUPAC Name
The IUPAC name for a polysubstituted benzene is constructed by combining the substituent prefixes, numbers, and benzene ring name.
For example, the IUPAC name for the compound with the following substituents:
- Methyl
- Ethyl
- Chlorine
Would be:
1-chloro-2-ethyl-3-methylbenzene
5. Exceptions and Special Cases
There are some exceptions and special cases to the IUPAC naming rules:
- Ortho, meta, and para prefixes: These prefixes are used to indicate the relative positions of two substituents on a benzene ring. Ortho indicates adjacent positions, meta indicates positions separated by one carbon atom, and para indicates positions opposite each other on the ring.
- Common names: Some polysubstituted benzenes have common names that are still widely used, such as toluene (methylbenzene) and xylene (dimethylbenzene).
Top FAQs
What are the general rules for naming polysubstituted benzenes?
The IUPAC guidelines for naming polysubstituted benzenes involve identifying and prioritizing substituents, numbering the benzene ring, and combining the substituent prefixes, numbers, and benzene ring name.
How do you determine the priority of substituents in IUPAC naming?
The priority of substituents is determined based on the atomic number of the atoms directly attached to the benzene ring. Higher atomic number atoms receive higher priority.
What is the significance of numbering the benzene ring in IUPAC naming?
Numbering the benzene ring allows for the unambiguous identification of the position of substituents on the ring. The numbering system is based on the lowest possible set of numbers for the substituents.
Are there any exceptions or special cases to the IUPAC naming rules?
Yes, there are some exceptions and special cases to the IUPAC naming rules, such as the use of prefixes like “ortho,” “meta,” and “para” for certain disubstituted benzenes.